Why Is Iron in Water a Problem?
Iron is one of the most common contaminants found in groundwater and well water. While not usually harmful in low concentrations, excessive iron in drinking water causes:
Metallic taste and unpleasant odor
Yellow, brown, or reddish stains on laundry and fixtures
Clogging of pipelines and appliances
Promotion of iron bacteria growth
Discoloration of drinking water
According to WHO and BIS 10500 standards, the maximum acceptable limit for iron in drinking water is 0.3 mg/L. Above this, treatment is essential.
What Forms of Iron Are Found in Water?
Understanding the form of iron in your water helps in selecting the right removal method. The main types are:
| Iron Type | Appearance | Removal Difficulty | Notes |
|---|---|---|---|
| Ferrous (Fe²⁺) | Clear water iron | Hard to remove without oxidation | Dissolved form |
| Ferric (Fe³⁺) | Reddish or yellowish particles | Easily removed by filtration | Oxidized form |
| Organic Iron | Brownish color | Requires special treatment | Bound to humic substances |
| Iron Bacteria | Slime-like film | Requires disinfection & filtration | Biogenic |
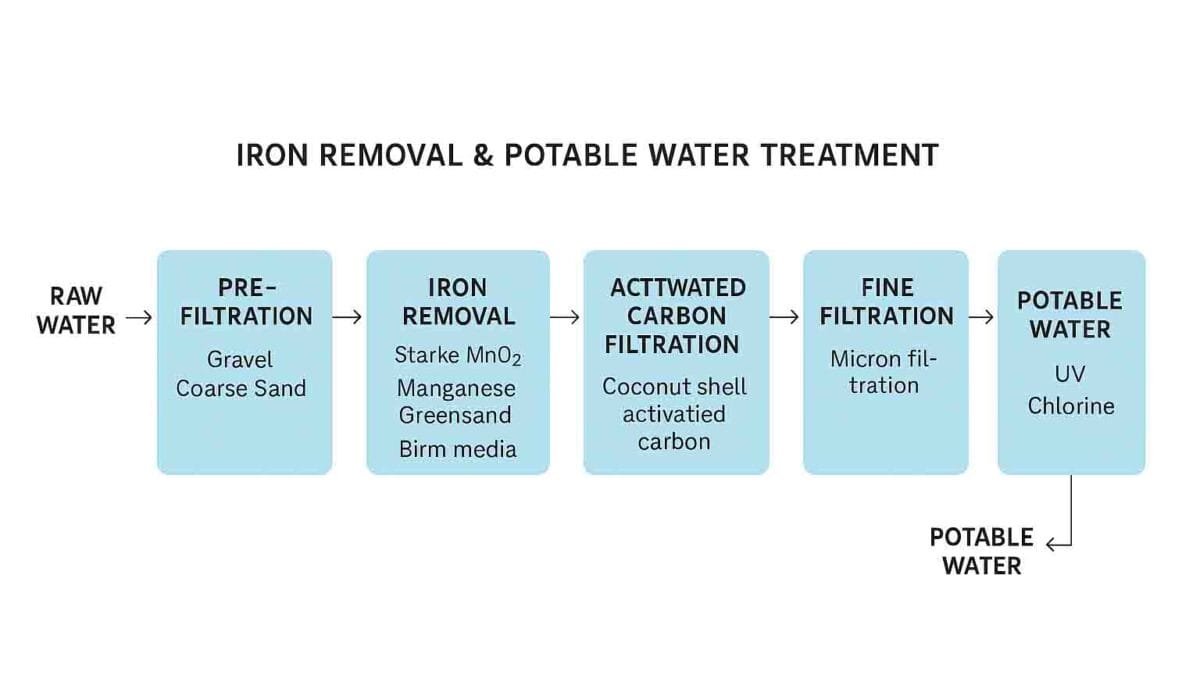
How to Remove Iron from Water: Proven Step-by-Step Process
Here’s a professional-grade, scalable solution to remove iron and make the water potable:
1. Pre-Filtration (Sediment Removal)
Before targeting iron, remove suspended solids that can clog the iron removal media.
Media Used: Coarse sand, gravel
Function: Remove turbidity, silt, and large particles
Placement: First layer in multi-media filtration systems
2. Iron Removal Stage (Main Step)
Depending on the form of iron, choose one or more of the following media:
✅ Starke MnO₂ (Starmnox)
High-purity manganese dioxide
Removes iron, manganese, and hydrogen sulfide
Works in wide pH range (6.2–8.5)
No chemical regeneration needed
Bulk density: ~1.5 g/cm³
✅ Manganese Greensand
Coated sand with manganese oxide
Removes Fe²⁺ and Mn²⁺ via oxidation
Requires regeneration with KMnO₄ (Potassium Permanganate)
✅ Birm Media
Lightweight catalytic media
Efficient for water with sufficient dissolved oxygen
No regeneration required
Not suitable for high iron loads or low pH
Tip: Use oxidation methods like aeration or chlorine dosing before filtration if water contains high levels of ferrous iron.
3. Oxidation (If Needed)
Oxidation converts soluble ferrous iron into filterable ferric iron.
Common Oxidation Methods:
Air Injection Systems (Venturi ejectors, diffused aerators)
Chlorine Dosing + Contact Tank
Ozonation or Hydrogen Peroxide for high iron/manganese water
Bonus Benefit: Oxidation also helps in killing iron bacteria.
4. Activated Carbon Filtration
After iron is removed, further polish the water to eliminate:
Chlorine residues
Odors
Organic impurities
Recommended Media:
Coconut Shell Activated Carbon
High adsorption capacity
Enhances taste and clarity
5. Fine Filtration (Micron Filter)
Micron filters provide a final polish before water is disinfected or consumed.
Rating: 5 to 1 micron
Function: Remove fine suspended solids
Material: Pleated PP, spun or cartridge filters
6. Disinfection (Final Step)
Even if water is iron-free and clear, it must be disinfected to kill pathogens.
Disinfection Options:
UV Sterilization: Kills bacteria & viruses instantly
Chlorination: Long-lasting protection, especially for storage tanks
Ozonation: Strong oxidant with no residual taste
Note: If chlorine was used for oxidation, ensure proper neutralization using activated carbon or chemical dosing before drinking.
How to Test for Iron in Water?
You can perform lab testing or a quick field test using iron test kits.
Simple Field Test Method (Ferrous Iron):
Take a sample of raw water in a transparent glass.
Add a few drops of household bleach (sodium hypochlorite).
Wait 5 minutes.
If water turns yellow to reddish brown, it has iron.
For accurate results, lab testing should measure:
Total Iron (mg/L)
Dissolved Iron (Fe²⁺)
pH level
Turbidity (NTU)
Where Are Iron Removal Filters Used?
Starke’s iron removal media is used in:
Domestic water filters
Industrial process water systems
Commercial RO pretreatment
Municipal and rural drinking water plants
Borewell and well water filtration
Why Choose Starke Filter Media?
Starke offers certified, high-performance filtration media trusted by water professionals in over 20 countries.
✅ Key Advantages:
AWWA/IS-certified media
High-purity MnO₂ with 80–85% minimum MnO₂ content
Technical guidance for system sizing and backwashing
Custom packaging & prompt global dispatch
Sample Design Flow for Iron Removal System
| Stage | Media | Flow Rate (m³/hr) | Bed Depth (mm) | Remarks |
|---|---|---|---|---|
| Pre-Filter | Coarse Sand | 10 | 600 | Removes turbidity |
| Iron Removal | Starke MnO₂ | 10 | 800 | Removes Fe²⁺/Fe³⁺ |
| Carbon Filter | Coconut Carbon | 10 | 600 | Removes chlorine, organics |
| Micron Filter | Cartridge 5 µm | 10 | — | Final polish |
| Disinfection | UV or Cl₂ | 10 | — | Kill pathogens |
FAQs: Iron Removal from Water
Is iron in drinking water harmful?
At high levels, it affects taste, color, and appliances. Over 0.3 mg/L, it must be treated.
Can boiling remove iron?
No. Boiling concentrates iron further and cannot oxidize dissolved forms.
What’s better: MnO₂ or Birm?
MnO₂ is more effective for high iron and broader pH range. Birm is economical for low iron and high oxygen conditions.
Do I need a water softener after iron removal?
Only if hardness is high (>300 ppm). Iron removal and softening are separate processes.
Final Thoughts: Turn Your Raw Water into Pure, Drinkable Water
Iron-contaminated water is a widespread issue, but with the right treatment system, it can be completely resolved. Whether you’re managing a borewell, industrial process, or rural supply, Starke Filter Media provides globally trusted solutions tailored to your water quality.
Contact Us
Email: info@starkefiltermedia.com
Website: www.starkefiltermedia.com